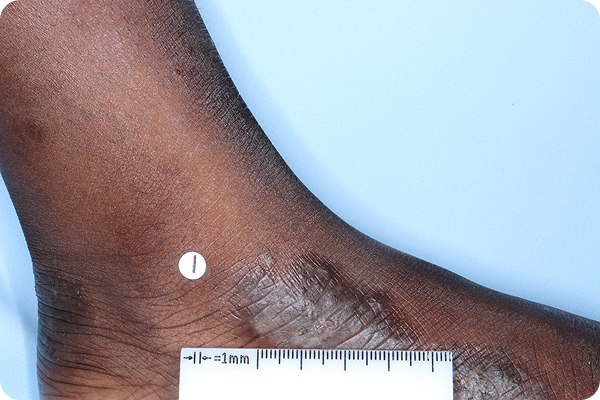
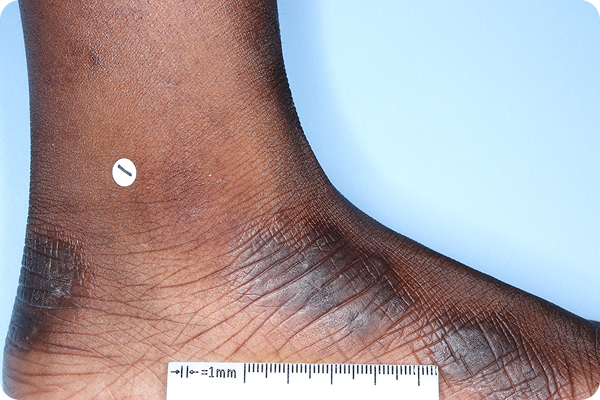
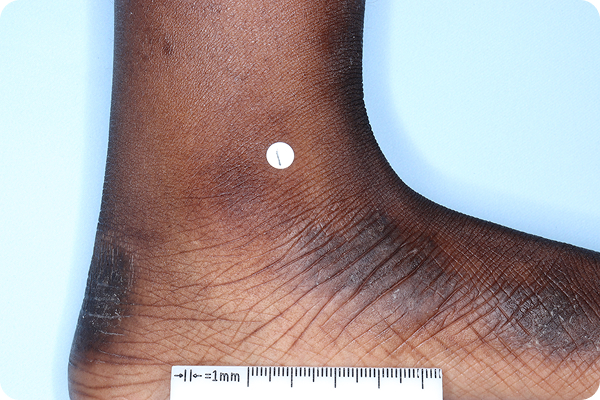
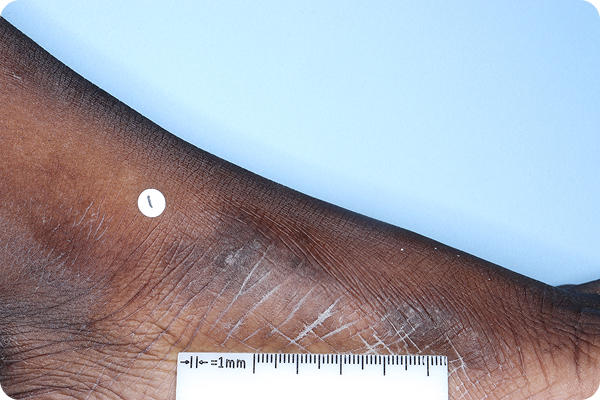
References: 1. VTAMA (tapinarof) cream, 1%. Prescribing Information. Organon; 2024. 2. Silverberg JI, Eichenfield LF, Hebert AA, et al. Tapinarof cream 1% once daily: significant efficacy in the treatment of moderate to severe atopic dermatitis in adults and children down to 2 years of age in the pivotal phase 3 ADORING trials. J Am Acad Dermatol. 2024;91(3):457-465. 3. Simpson E, Silverberg JI, Bissonnette R, et al. Rapid and early onset of itch relief with tapinarof cream 1% once daily in two pivotal phase 3 trials in adults and children down to 2 years of age with moderate to severe atopic dermatitis. Oral presentation at: European Academy of Dermatology and Venereology; October 11-14, 2023; Berlin, Germany. 4. Organon DOF [ADORING 1&2 Pooled Posthoc; Jan 2025]. 5. Bissonnette R, Stein Gold L, Kircik L, et al. Skin clearance, treatment response off-therapy, and safety of tapinarof cream 1% once daily: results from ADORING 3, a 48-week phase 3 trial in adults and children down to 2 years of age with atopic dermatitis. Poster presented at: Fall Clinical Dermatology Conference; October 24-27, 2024; Las Vegas, NV. 6. Stein Gold L, Del Rosso J, Ehst BD, et al. Tapinarof cream 1% once daily was well tolerated for the treatment of adults and children down to 2 years of age with moderate to severe atopic dermatitis across two pivotal phase 3 trials. Poster presented at: American Academy of Dermatology; March 8-12, 2024; San Diego, CA. 7. Organon DOF. ADORING Patient Images. 2024. 8. Organon DOF. DMVT-505-3103. 2024. 9. Hebert AA, Del Rosso J, Johnson SM, et al. Patient and parent/caregiver satisfaction with efficacy and cosmetic elegance of tapinarof cream 1% once daily in a long-term extension trial in adults and children down to age 2 years with atopic dermatitis. Poster presented at: Fall Clinical Dermatology Conference; October 24-27, 2024; Las Vegas, NV. 10. Silverberg JI, Boguniewicz M, Quintana FJ, et al. Tapinarof validates the aryl hydrocarbon receptor as a therapeutic target: a clinical review. J Allergy Clin Immunol. 2024;154(1):1-10. 11. Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137(10):2110-2119. 12. Sutter CH, Azim S, Wang A, et al. Ligand activation of the aryl hydrocarbon receptor upregulates epidermal uridine diphosphate glucose ceramide glucosyltransferase and glucosylceramides. J Invest Dermatol. 2023;143(10):1964-1972. 13. Organon DOF. The effect of GSK2894512 on the Th2 polarization of human CD4+ T cells and on IL-8 release from TLR2-stimulated keratinocytes. 2015. 14. Bissonnette R, Poulin Y, Zhou Y, et al. Efficacy and safety of topical WBI-1001 in patients with mild to severe atopic dermatitis: results from a 12-week, multicentre, randomized, placebo-controlled double-blind trial. Br J Dermatol. 2012;166(4):853-860.