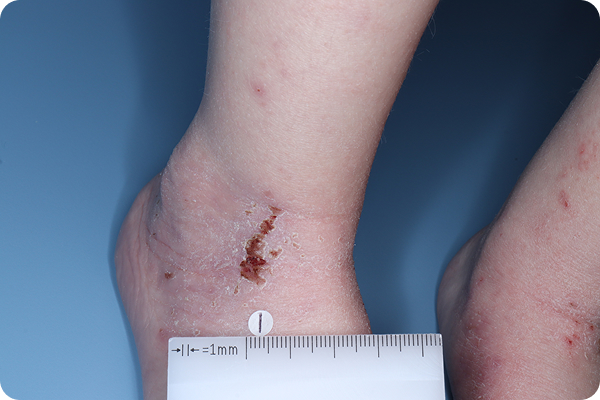
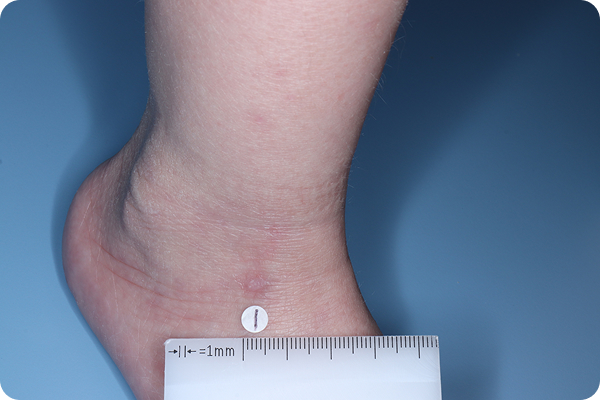
VTAMA cream is a once-daily, steroid-free treatment for atopic dermatitis in patients down to 2 years of age1,2
VTAMA cream demonstrated:

Powerful skin
clearance2
In the 8-week pivotal studies:
- Up to 46% of patients achieved clear or almost clear skin compared to 18% on vehicle*2
In the 48-week, open-label, long-term extension study:
- The majority of patients (~52%) entered with or achieved complete disease clearance (vIGA-AD™=0) at least once†5
- Patients had ~80 consecutive treatment-free days on average during the first treatment-free interval after achieving complete disease clearance5

Rapid itch
relief2-4
- Statistically significant itch relief as early as Day 2 in the pivotal studies2-4

Favorable safety and
tolerability across
56 weeks2,5
- A favorable and consistent safety profile in both the 8-week pivotal studies and the 48-week LTE study, and was well tolerated, even on sensitive skin areas2,5,6
vIGA-AD, Validated Investigator Global Assessment for Atopic Dermatitis. vIGA-AD is a trademark of Eli Lilly and Co.
*In ADORING 1 and ADORING 2, vIGA-AD success, defined as vIGA-AD=0 or 1 and ≥2-grade improvement from baseline at Week 8, was achieved by 45% and 46% of patients on VTAMA cream vs 14% (P<0.0001) and 18% (P<0.0001) of patients on vehicle, respectively.2
†Including patients who entered with vIGA-AD=0 (n=58) and patients entering with vIGA-AD≥1 who achieved vIGA-AD=0 at least once during the open label, LTE (n=320).5