

Help your patients relieve their itch, so they can get back to more of life’s good stuff.2-4
In clinical studies, patients with atopic dermatitis that were treated with VTAMA cream reported rapid itch reduction compared to vehicle cream–treated patients.2-4
‡P=0.0366.
§P=0.0015.
PP-NRS, Peak Pruritus-Numeric Rating Scale.
*Daily PP-NRS scores were recorded in a diary self-completed by patients ≥12 years of age and completed by caregivers for patients 2-11 years of age.2,3
†Patients with baseline PP-NRS score ≥4 who achieved ≥4-point reduction in the PP-NRS from baseline.2
Improvements in daily PP-NRS scores seen with VTAMA cream vs vehicle continued through the first 2 weeks (-3.0 vs -2.0 and -2.9 vs -1.8 at Day 14, in ADORING 1 and ADORING 2, respectively) and through Week 8 in both studies.2-4
PP-NRS, Peak Pruritus-Numeric Rating Scale.
*Daily PP-NRS scores were recorded in a diary self-completed by patients ≥12 years of age and completed by caregivers for patients 2-11 years of age.2,3
†In ADORING 1, greater reduction in itch was observed with VTAMA cream vs vehicle as early as Day 1, 24 hours after initial application: -1.2 (2.2) vs -0.9 (2.0); improvements continued through Day 14: -3.0 (2.8) vs -2.0 (2.4), with continued improvement through Week 8. In ADORING 2, greater reduction in itch with VTAMA cream vs vehicle was observed as early as Day 2. The 95% confidence interval (CI) for the daily mean treatment difference in PP-NRS scores excluded 0 as early as Day 2 in ADORING 1 (-1.1, -0.2).3
The most common adverse reactions (incidence ≥1%) in patients with atopic dermatitis treated with VTAMA cream were upper respiratory tract infection, folliculitis, lower respiratory tract infection, headache, asthma, vomiting, ear infection, pain in extremity, and abdominal pain.
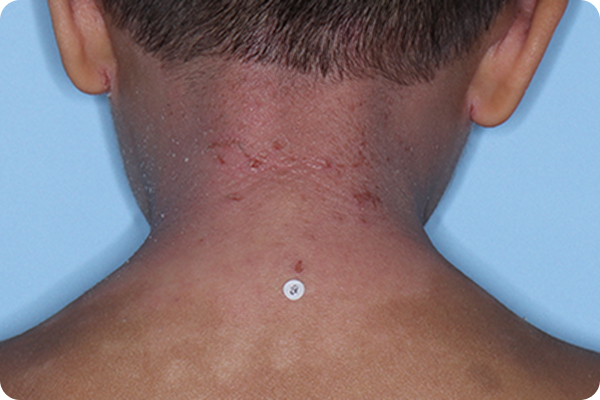
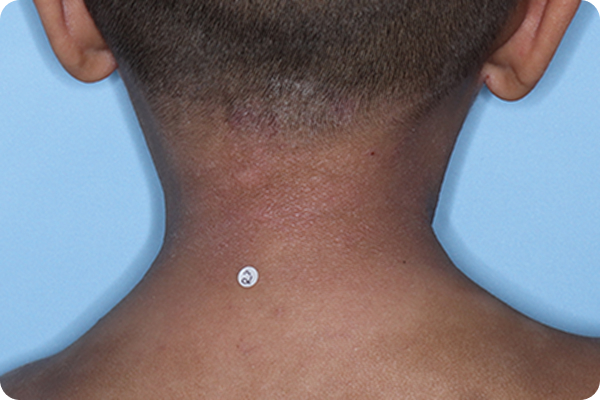
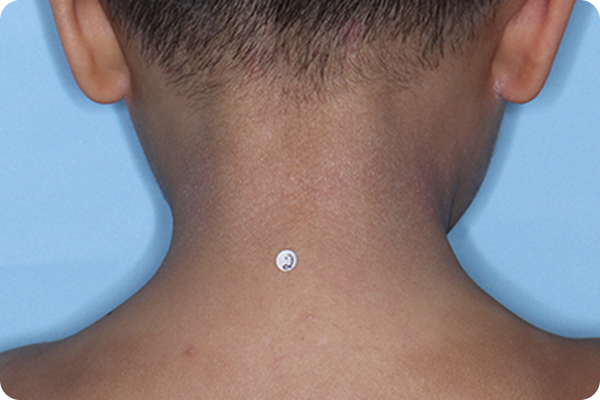
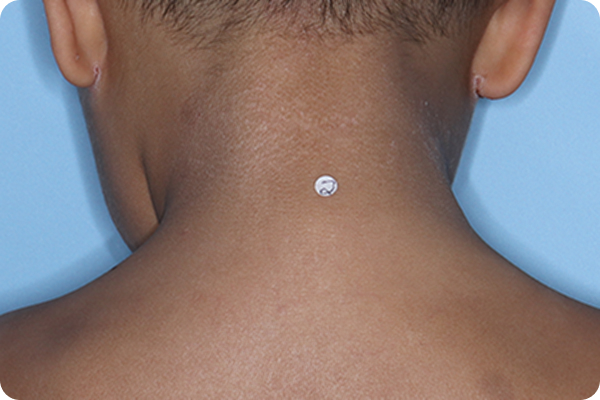
Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD): measure of AD severity with scores ranging from 0 (clear) to 4 (severe). vIGA-AD is a trademark of Eli Lilly and Co.
Eczema Area and Severity Index (EASI): measure of disease severity that incorporates body surface and lesion intensity into a composite score from 0 to 72, with higher scores indicating more severe disease.
Patient Oriented Eczema Measure (POEM): 7-item questionnaire to measure eczema severity focusing on the disease as experienced by the patient. Scores range from 0 to 28, with higher scores indicating more severe disease.
Peak Pruritus-Numeric Rating Scale (PP-NRS): patients are asked to rate their worst itch in the past 24 hours on a scale of 0 (no itch) to 10 (worst itch imaginable).
*Please note that these photographs are representative of one target lesion on individual patients treated with VTAMA cream during the pivotal clinical studies.
†Primary endpoint success defined as vIGA-AD score of 0 (clear) or 1 (almost clear) and ≥2-grade improvement from baseline at Week 8.2
Indications: VTAMA® (tapinarof) cream, 1% is an aryl hydrocarbon receptor agonist indicated for:
Adverse Events: In plaque psoriasis, the most common adverse reactions (incidence ≥1%) were: red raised bumps around the hair pores (folliculitis), pain or swelling in the nose and throat (nasopharyngitis), skin rash or irritation, including itching and redness, peeling, burning, or stinging (contact dermatitis), headache, itching (pruritus), and flu (influenza).
Adverse Events: In atopic dermatitis, the most common adverse reactions (incidence ≥1%) were: upper respiratory tract infection, red raised bumps around the hair pores (folliculitis), lower respiratory tract infection, headache, asthma, vomiting, ear infection, pain in extremity, and stomach-area (abdominal) pain.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Before prescribing VTAMA cream, please read the Prescribing Information
